Level: Alchemist
Reading time: 20 minutes (if you are lucky)
Question: Time? Things are considered tempered when you take a test strip cool it and it has the characteristics you want. My question is does this happen rapidly, say 95% of the fat is in “V” at 10 mins, or is “tempering” when say 50% of the crystals are in the correct form. Does extending the time at temperature to say a day or 2 get significantly closer to 100% in V? Does this make a difference? You probably reach a point of diminishing returns, just curious how much science has been applied to this voodoo…
Funny you should mention voodoo. As I am going to take this into the exact opposite direction and define tempering in technical terms.
It’s worth noting that as I think of all I want to convey I feel like the philosopher who has attained enlightenment from the study of a butterfly wing and now wants to convey it to everyone….and takes page after page explaining it and ultimately fails because you usually can’t explain deep concepts quickly or easily. Regardless….onward.
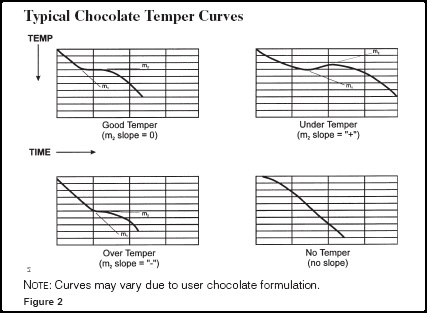
Chocolate is tempered properly when the inflection point of the of a temperature vs time cooling plot has a slope of zero.
And I suspect that doesn’t mean or explain anything to most of you. If you have a look around the internet you will find this old school image pretty quickly.
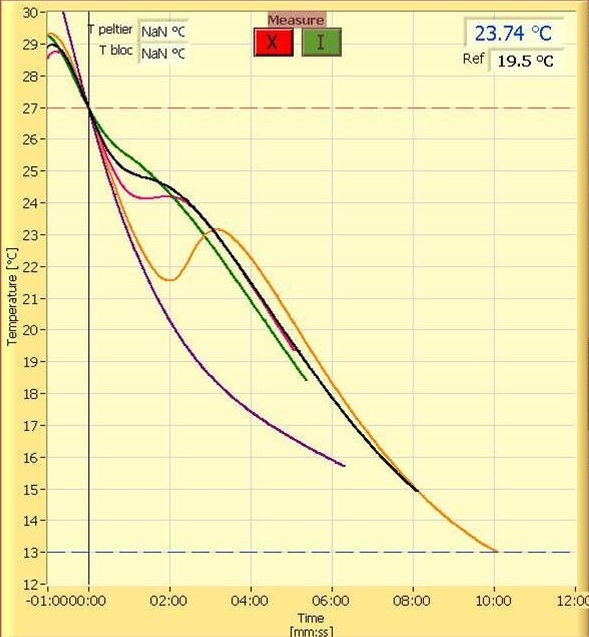
Digging a bit deeper and you can find something like this that shows real data in a form more of you can relate to.
In both cases they are showing plots of chocolate in different stages of temper, from untempered all the way to over tempered.
And I want you to read that last line again. There is something very important there that takes many people many years to realize. The first epiphany as it were. Over tempered. Chocolate can be over tempered. Understandably a large number of people think you want 100% temper. All Type V crystals. But that isn’t the case. You want a balance.
So that answers a part of your question. Or more to the point, it sheds some light on some of the assumptions you have made about tempering. That it is an end state. Sort of like absolute zero. And maybe it is. The key here is understanding that IF the perfect temper is analogous to absolute zero, then your goal is NOT absolute zero, but some temperature above that.
Try and let that sink in. I’ll try and explain why that is at the end.
But before we get deep into all the revelations that those plots can give us, let’s take a few moments and talk about what you are seeing there.
WARNING – SCIENCE AHEAD
Pretty much all of tempering is predicated on the concept of crystallization.
The following concept is one you need to wrap your head around.
Solidifying fat crystals give off heat in the same way dissolving salt crystals take in heat. These are the two sides of the same coin or concept.
That doesn’t make sense? This puts it into tangible terms.
If you make ice cream the old fashion way, you will recall you mix ice with salt. Remember that? Do you know why? Solid salt is just sitting there. It has low energy. Salt in solution is moving around. It has more energy. That energy has to come from somewhere. In the case of your ice cream and ice, it comes from the temperature of the system. Ice cream, ice and salt separate are all at 32 F. If the salt needs energy to dissolve, then it has to steal it from somewhere so it takes it from the only place it can; the temperature of the ice cream and ice. The result is that those temperatures drop to something under 32 F as the salt dissolves and your ice cream solidifies.
Got that? The whole point is to melt a crystal it has to take in heat. Therefore the opposite is also true. To form a crystal, heat is given off. This heat is called the heat of crystallization. To go all science and geeky on you:
“Heat of crystallization or enthalpy of crystallization is the heat evolved or absorbed when one mole of given substance crystallizes from a saturated solution of the same substance.”
Let’s bring this back to chocolate and set the stage. You are in the process of tempering your chocolate. You have either made or introduced seed to your untempered chocolate. For the sake of discussion, let’s call it 31 C. You pour it up into your mold and it starts to cool and eventually set up.
If we peek behind the curtain, and can monitor the temperature and crystals we are going to see the temperature start to drop. That is just the natural cooling of the chocolate. Energy is being given off to the atmosphere or maybe your refrigerator. At the same time, the seed of Type V crystals are going to start propagating or growing.
And now the fun part (did I mention I’m a retired chemist who finds this fun?).
We can make a prediction. As the crystals start to form we know (see above) that they are going to start giving off heat to the surround chocolate. And what is that going to do?
Very good. The temperature is going to rise.
And if we record those temperatures and the time, and then plot them, what is it going to look like? It is going to be a decreasing line (the temperature is falling initially because it is in the refrigerator or whatever) then, (oh, I’m so excited) since those crystals are giving off heat, (I’m virtually giddy here) the line is going to level off or maybe (shaking with geek excitement) even go back up! Then, once all the crystals are formed and no more heat is being evolved (yep, pulled in a science word there) the temperature will continue to drop to whatever the ambient temperature is.
Do you have that image in your head? Hopefully, it looks a LOT like this:
Which, wow, looks pretty much identical to the plot at the beginning for properly tempered chocolate!
Science!!! (giddy again….calming down…. )
Okay, I’ve calmed down for the moment. Hopefully you have the concepts down and we can talk about what they mean.
The first thing I want to address is that a zero slope (that flat spot) is nothing special. It is empirical meaning that it is just lucky coincidence that the majority of we humans like our chocolate when it is tempered at a zero inflection.
If you go back to the top and have a look at the curve of untempered chocolate you will notice it is basically a straight line pointing down.
“But alchemist, even bloomed chocolate has crystals. Type III, IV and V. Don’t they have a heat of crystallization and wouldn’t they cause some heat rise?”
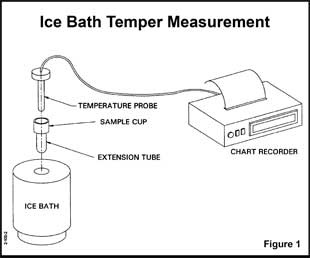
I am proud of you for making that connection. It is 100% true. IF…and this is where I didn’t play straight with you…we were truly measuring the temperature of your chocolate as it set up. But that is not what those plots are from. They are actually from a special setup that measures the degree of temper in a chocolate. It basically looks like this:
This image and the text below on It’s workings is from Ticor systems
“Their operation is quite simple. A cup-like opening at one end of a long metallic (copper) tube is filled with a chocolate sample. The other end of the tube is inserted in a mixture of ice and water held in an insulated container. A temperature probe (thermocouple in early versions) is then inserted into the chocolate. Since the chocolate sample is above 85°F and the sample holder is attempting to reach the temperature of the ice bath (a questionable 32°F), the chocolate gives up heat to the holder and is cooled to solidification.”
The key difference here is the ice bath and that in a degree of temper analysis you are forcing the chocolate and temperature down quickly along a reproducible path (because is a constant temperature and the system is insulated). The consequence of this is that you can see deviations easily and you are literally forcing the chocolate to solidify without crystallizing. Because no crystallization is happening, no heat is given off and no rise in temperature is noted.
Got it? Good!
And now we can talk about some of those bits of enlightenment that fall out of these graphs.
If you look at the 2nd graph above with all the overlaid plots you see a spectrum of non-tempered curves all the way to over tempered. This means tempering is a continuum and what falls out of that is that tempering is not a discrete state but a process and because we can have a full range of tempers we can have variables that we can adjust in a predictable manner to give us those final degrees of temper.
What are those variables? Well one big one should be obvious. The amount of Type V seed you have present and the original question hints at that. And that makes sense. The more seed you have, the more energy that is given off in a set amount of time and the more the cool curve tips up. Likewise, if you don’t have enough, it doesn’t tip up enough, the slope of the line doesn’t make it to zero, and you have under tempered chocolate, and if it is under tempered too much, it can bloom.
So, is time something you can manipulate? Will more time (2 days?) give you a higher degree of temper because more seed is formed? It certainly seems like it might be, and it can be, but it turns out that it isn’t practical. And the key is understanding that time is only helpful if it is your only variable. And in many cases it isn’t.
If you are tempering from scratch it is useless. Why? Because when you bring your chocolate down to 80 F or so and form seed, how much you form is varies with how fast you reduce the temperature and the ambient temperature. Without being able to control those exactly, you will have different amounts of seed (which you have no way of knowing) and that basically means you are starting from a different point in the tempering process each time so you can’t get to the degree of temper by measuring time.
If that isn’t clear, think of it as getting a car up to 60 mph (perfect temper, zero slope inflection) from a random unknown speed with only a stopwatch. It can’t be done. There are too many variables. How fast are you going (how much seed initially), how much are you accelerating and how do you measure it (what is the ambient temperature) and are there hills that you have to go up or down (how much heat loss do you have).
This is why hand tempering can be a series of failures until you both get a feel for how fast 60 mph is and you get used to the road (i.e. your techniques and conditions get VERY reproducible).
So what are you to do? You use some form of seed. And a tempering machine or water baths if you can. Neither is magical. They are nothing more and nothing less than variable control and management.
When you use seed or silk you starting with a known amount of seed, and better yet (analogy shift warning) your speed IS 60 mph and you just have to maintain it by watching the speedometer (your temperature).
If you want a lower degree of temper, you use less seed/silk. If you want I higher degree of temper, you use more. That simple.
So, it was probably hard to miss that I mentioned both seed and silk. The distinction is that the former is tempered chocolate and the latter is tempered cocoa butter. And they produce different tempers. Not just different degrees of temper, but actually different types of temper.
S. Beckett (The Science of Chocolate) notes this:
The temperature at which the inflection on the curve occurs is very important. The higher the temperature the more mature the crystallization and the higher the temperature at which the chocolate can be used for moulding and enrobing.
Silk makes for a more mature crystallization and is my personal favorite way to temper (video of that coming up). You can see this by how it is used. You mix it in at around 93-94 F. You use significantly less (0.5-1%) than you would tempered chocolate (20-30% many people recommend) and it propagates very fast through the chocolate, setting up quickly and with a particularly high resistance to blooming. And if you were to run a cooling curve on it you would see it produces the inflection point at a higher temperature correlating with a more mature crystal.
Is this all helpful day to day? I personally think it is since I wrote it. How? I think 90% of the help it gives is taking some of the mystery out of tempering (after you reread it a few times). It is just variables and if you grasp that, you can start to look for variables in your process and eliminate them or at least identify them when something goes awry and you chocolate blooms.
Can your or should you set up your own temper meter? If you find it fun and like the hard data, by all means do it. It isn’t hard. It is basically a thermometer and an insulated ice bath. You will probably have to play around a little with your sample size to get a flat line for untempered chocolate but that is about it. But of course you don’t have to. Hell, I LOVE this stuff and have not done it. But you can.
Oh, one more thing.
Remember I said I would explain why you don’t want an absolute temper? It’s really kind of simple. Mostly it is because you are a person eating chocolate and not a chemist striving for a perfect beautiful crystal. You are not looking for perfection in structure. You are looking for perfection in mouth feel. You are looking for an experience. You are looking for that perfect state where the chocolate is smooth and melts easily in your mouth. That point of state that allows maximum release of flavor and enjoyment. You are looking for the perfect tipping point where it is almost bloomed and almost too hard.
You are looking for balance.
I hope that was enough science to this voodoo we call tempering.
Welcome to the art AND science of chocolate making.