Level: Apprentice
Reading Time: 8 minutes
In one of your articles you mentioned you like silk tempering more than chocolate seed tempering because of the strength of type V crystals in tempered cocoa butter. Can a chocolate tempered with chocolate seed have a chance of blooming because of the seed? I'm not sure if my last question made any sense but all I'm trying to understand is why chocolate seed crystals are not as good as silk seed crystals?
Yes, I very much like silk tempering. It is robust and highly predictable. I’m a retired chemist. It is hard to describe to you just how much tempering disturbs me. I know it is the bane of many of your existences but that isn’t what I mean. I mean it bends to the point of breaking so many rules about crystal formation that I am sort of in awe that it EVER works. Remember, tempering is nothing more than stacking cocoa butter molecules in a certain way. When you stack or arrange molecules in an orderly fashion the result is called a crystal. In tempering we want Type V crystals.
When we had to form crystals in chemistry lab there were a couple universal rules. Treat everything carefully. Don’t jostle them. Give them lots of time to form. Keep the temperature even. Let them grow very slowly for great crystals.
I grew this crystal. It is about half the size of my pinkie nail. It took me about 2 weeks of very careful tending.
I also had a whole bunch that I rushed, nudged and generally didn’t handle well.
But what do we do with chocolate when we temper it? We stir it, and scrape it and manipulate it. Everything that I was taught NOT to do.
Yet it works.
Sometimes. And in fairness, it tends to fail when you flaunt some of the chemistry lab rules.
You wanted to know why silk is better than seed.
It is all about purity and size. Silk crystals are purer and larger. That is the basic answer. If you want to know more, read on.
Aside from disturbing crystals while they are growing the other thing that can mess up crystal growth are impurities. When you grow crystals in the lab you use a pure substance and a solvent. They stack up neat and nice. You take special care to make sure very little else is there to disturb what is call the matrix.
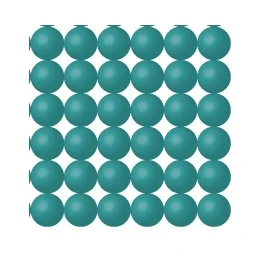
As an example, pretend we are stacking up balls. It’s a pretty good analogy really. I good crystal looks like this.
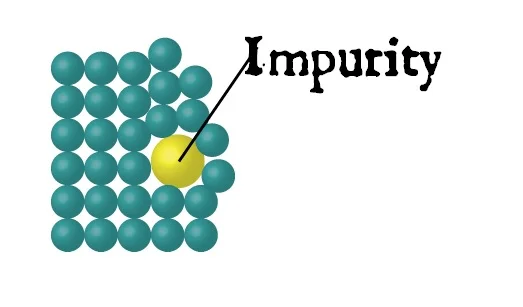
What happens if we put something else in there? An impurity. It is no longer neat and even.
The balls are just not able to form neatly around the impurity and it distorts the matrix. The result in many cases is a change in one of the physical properties of the crystals. The big three are stability, melting point and color. Can you see where I am going? It is great if you can, otherwise hold tight and I’ll explain it.
Atoms and most crystallizing molecules don’t actually look like balls. But they often act close enough that we can use the representation to make a point. Cocoa butter doesn’t look like round balls; not even close. And in all honesty you don’t really need to know what they look like. But I am still going to show you a closer approximation so you can have an inkling of appreciation what a special system chocolate is.
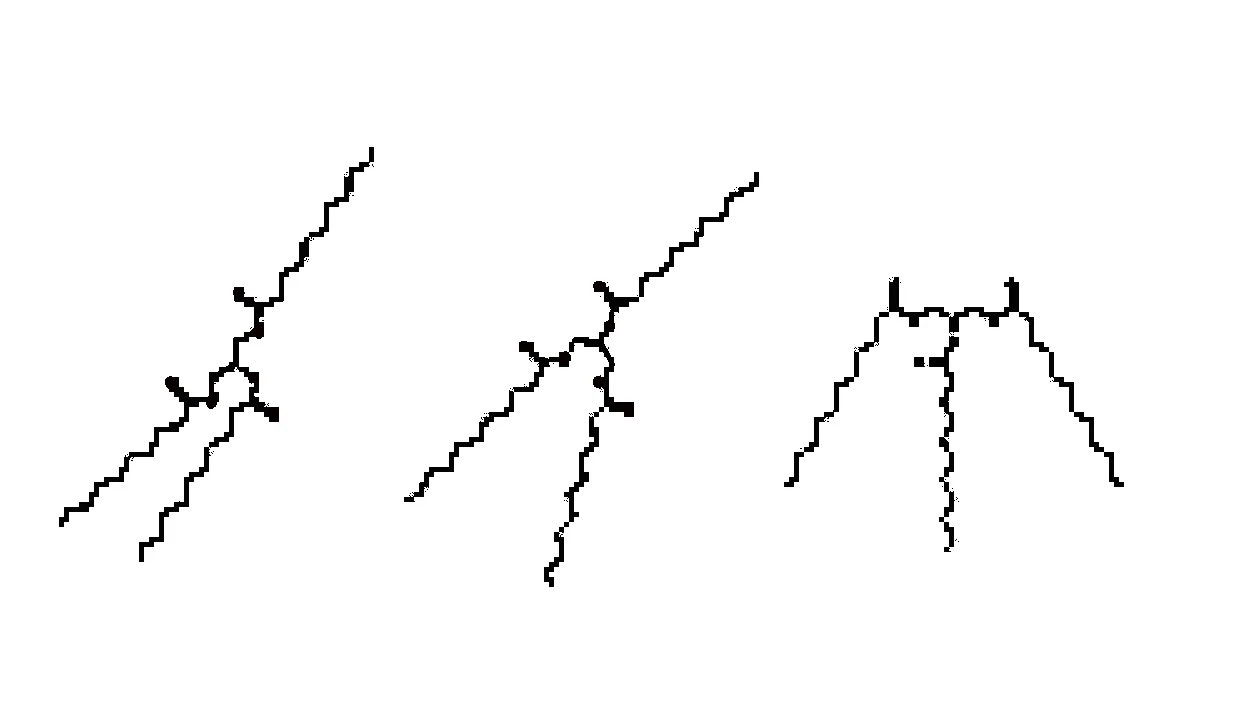
Instead of a ball you have this.
That doesn’t look too bad, does it? I bet you can picture stacking a bunch of those up nicely. But reality isn’t that neat and tidy. It’s just how we draw them for clarity. What is closer to reality (and still not super accurate) is that they can also look like any of these. Each leg tries to flip and turn trying to get away from the others.
I’m not going to delve any deeper in this direction right now for fear of overwhelming many of you. At some point I will. But what I want you to do is picture in your own mind how difficult those could be to stack up in an orderly fashion.
That is a bit tricky, isn’t it?
I recently spent 6 hours on a plane doing just that. Working out the huge number of ways they can stack together in 3D space. Twelve pages my brain hurt. I won’t subject you to that. Yet.
That takes care of the base cocoa butter molecules.
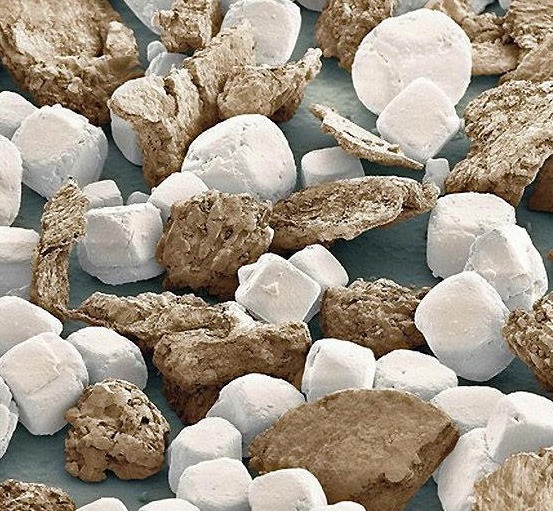
How about adding in some impurity? Say, like the sugar we add to chocolate? Again we show it as a nice round perfect sphere but it really looks like this.
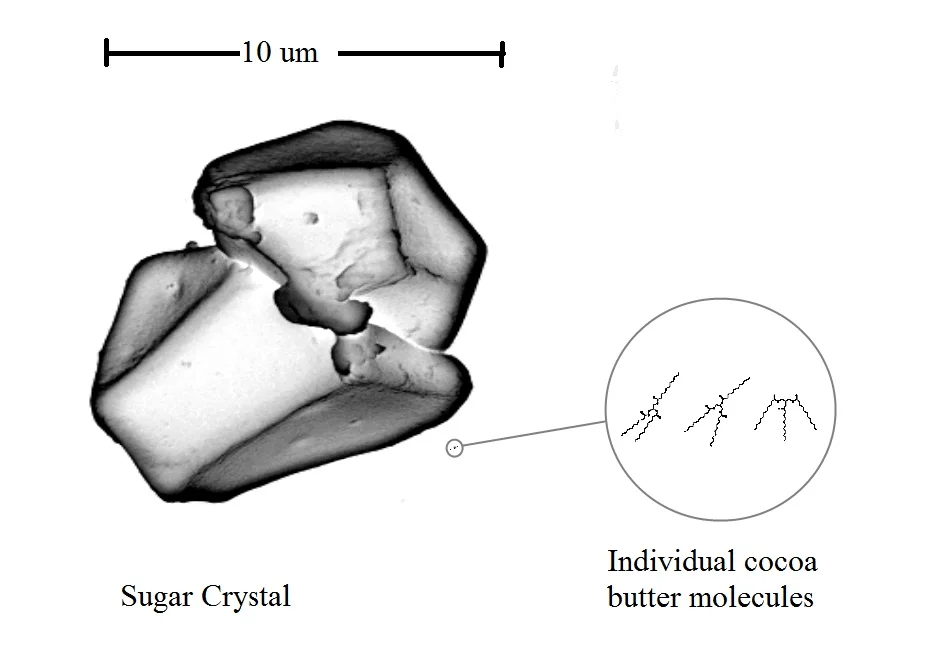
This is really what a 10 micron particle of granulated sugar looks like after it has been refined. It certainly isn’t a perfect cube nor is it a perfect sphere. A lot of the rough edges have been rounded off, but it is by no means a smooth surface. To top it off, in chocolate there are also cocoa particles in there. It sort of would look like this.
This is what the cocoa butter has to try and deal with.
Can you see how many varied surfaces there are and why I am amazed it even works? Each one of those makes it harder for the cocoa butter to stack up nicely. Why? It is about scale. Recall I mentioned size and purity matters. This is the beginning of why size matters. Have a look at how small the actually cocoa butter molecules are compared to the sugar crystal.
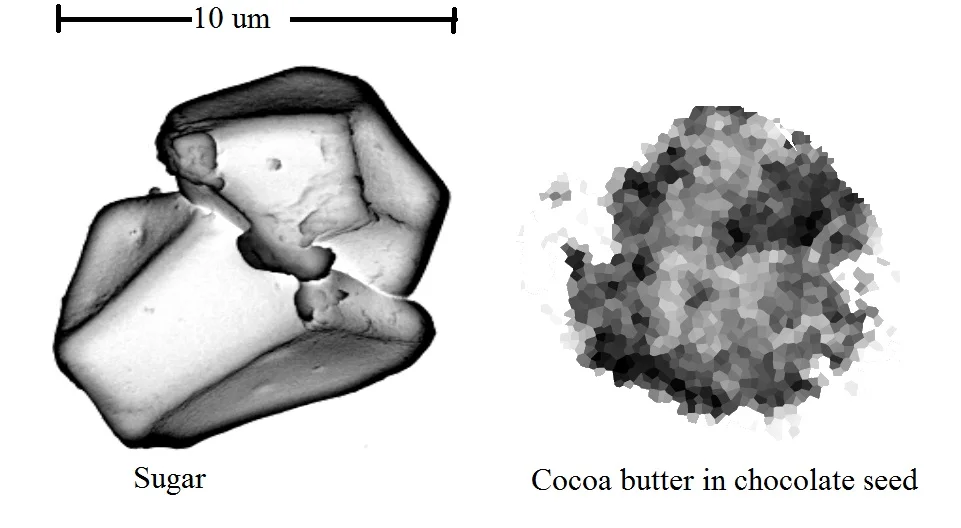
So when you are tempering from scratch or with seed, this is what cocoa butter has to contend with. The individual groups of crystals (called Spherulites) can only get so big before the sugar and cocoa pieces start to get in the way.
The seeds can only get so big before they get to unstable due to all the other things in the chocolate. That instability is why bloom happens easier in seeded chocolate.
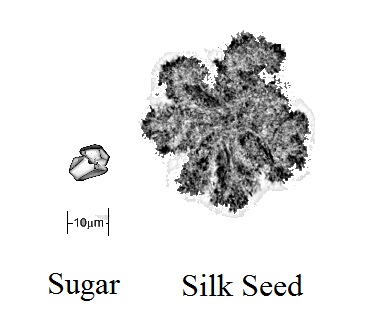
But silk is pure. It is just cocoa butter. The crystals can grow and grow and grow. Cocoa butter seed crystals are somewhere in the ballpark of 10 microns. Cocoa butter silk on the other had can grow to 40-50 microns. That may not sound like much but have a look at it compared to a sugar crystal.
What that means is that there is 25 times the surface area for loose cocoa butter molecules to find their places. It would be not true to say it was 25 times easier….but as analogies go, it works. In Silk, cocoa butter is the dominant structure by size. They can continue to stack together significantly easier. It basically is the elephant at the watering hole.
Remember that really nice crystal I grew? It was from a harvested seed. What I mean by that is that I picked through a bunch of crystals until I found a nice big perfect one. Then I let more material form on it…and it grew much faster. And is much harder than the small imperfect crystals.
Oh, there is one more point to make about the robust nature of Silk. It may seem the spherulites are a mere 5 times larger. That is just the dimension across. In volume, in three dimensions, that means they are 125 (5x5x5) times larger. That makes them MUCH more temperature resistant which is why you can use temperatures over 92 F when silk tempering (my personal best is 101 F, but that is not a recommendation or challenge). Think of two balls of ice; one the size of your hand, the other the size of a bowling ball. If you were to put both in warm water, the bowling ball sized ice sphere is going to last significantly longer than the small ice ball since there is so much more (125 times more ) ice present.
That is it.
Silk is just a pure, large cocoa butter seed crystal that allow more crystals to grow on it faster and is stronger, giving you a good strong temper.